Heat Capacity of Calorimeter
It is usually expressed as calories per degree in terms of the actual amount of material being considered most commonly a mole the molecular weight in grams. T 2 T 1 is the temperature difference before and after heating or cooling K.
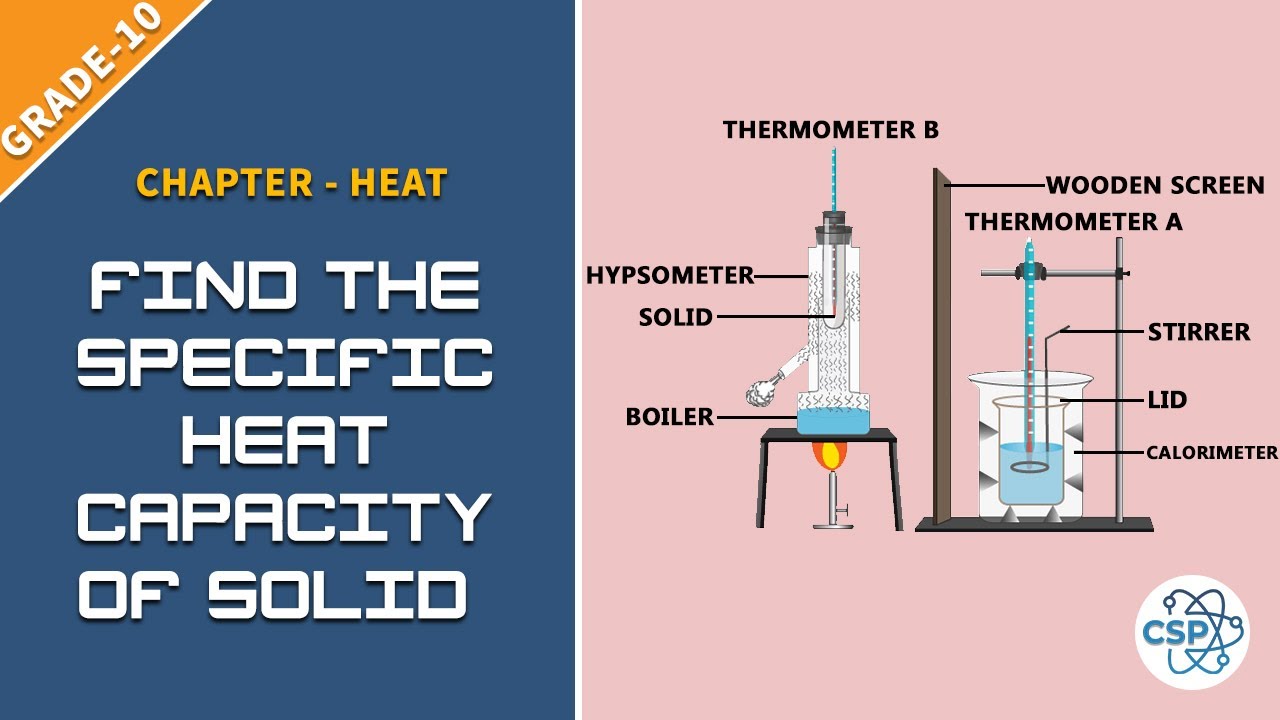
To Find The Specific Heat Capacity Of Solid By Using Method Of Mixtures See Class 10 Physical Properties Heat Science Experiments
Find the heat transferred to the calorimeter if the heat capacity of the calorimeter is 893 kJK.

. The specific heat capacity c Jkg K of tissue describes how much energy is required to change the temperature of 1 kg of tissue by 1 K 1C. If you have a specific heat capacity in Jg C then you need the mass of the substance in grams. Q is the heat absorbed or released by a material J.
Hier sollte eine Beschreibung angezeigt werden diese Seite lässt dies jedoch nicht zu. The specific heat also called specific heat capacity is the measure of the heat energy that a substance in a unit quality absorbs or releases when the. Recently with the development of the highly-functional polymeric material these thermal properties analysis needs are increasing dramatically.
A simple calorimeter just consists of a thermometer attached to a. Start your trial now. This is the amount of heat required to raise 1 gram of that substance by 1C.
Soc 1929 51 2738. This experiment is an extremely quick and relatively precise specific heat capacity test for a solid sample. This will require 2669 kJ of heat energy.
Thermochemistry determine the heat exchanged at constant pressure q m c T. Science Chemistry QA Library In a coffee-cup calorimeter 1200 mL of 10 M NaOH and 1200 mL of 10 M HCl are mixed. References Theory of Heat Maxwell James Clerk page 57-67 Westport Conn Greenwood Press 1970.
If you have it in Jkg C then you need the mass of the substance in kilograms. C is the specific heat of a material JgK. Calorimeter device for measuring the heat developed during a mechanical electrical or chemical reaction and for calculating the heat capacity of materials.
One type in widespread use called a bomb calorimeter basically consists of an enclosure in which the reaction takes place surrounded by a liquid such as water that absorbs. Calculating the limiting reactant the change in enthalpy of the reaction H rxn can be determined since the reaction was conducted under conditions of constant pressure H rxn q rxn moles of limiting reactant. The vessel is filled with water and the fuel is burned leading to the heating of the water.
Heat capacity ratio of heat absorbed by a material to the temperature change. 131 Specific heat capacity. Experiments were carried out using a MicroCal PEAQ-ITC calorimeter with MicroCal PEAQ-ITC Software version 13 both Malvern at 5 C in 20 mM Na 2 HPO 4 pH 75 150 mM NaCl or SPG buffer pH 50.
It uses the time-tested Parr 1108 style oxygen bomb and oval bucket in a compact calorimeter producing reliable results with good repeatability but differing from the 6400 Model in that the bomb and bucket. Assuming that all the solutions have a density of 10 gcm3 and a specific heat capacity of 418 JCg calculate the enthalpy change for the neutralization of HCl by NaOH. Both solutions were originally at 261C.
Say in a calorimeter a fixed amount of fuel is burned. Solution for Part A Calorimetry is a method used to measure enthalpy or heat changes that occur during chemical processes. 195 Degrees C Ending temperature 56 Degrees C Mass of calorimeter water after passing steam 0151Kg Mass of the condensed steam 0006Kg Heat gained by the water.
Expanded polystyrene polystyrene foam or styrofoam cups are often used as take-away coffee cups because the expanded polystyrene is a good insulator that is your coffee stays hot but you dont burn your fingers holding the cup. To calculate the energy required to raise the temperature of any given substance heres what you require. Anyone with access to a kitchen can do a form of this experiment and become a thermal physicist.
Thermal capacity of water. Heat capacity c 893 kJk. C is the specific heat capacity of water which is 1 calg C 1 calorie per gram per degree Celsius.
For example the lower specific heat capacity of fat compared to other soft tissue indicates that fat requires. M water is the mass of the water expressed in grams g. The actual value for the specific heat capacity of water is 4200 JkgC.
HClaq NaOHaq -- NaClaq H 2 Ol Energy. This same insulating property can be exploited to make a reasonably good calorimeter a device used to measure energy or heat change during. M is the mass of a material g.
The heat capacity in calories per gram is called specific heat. Two common calorimeters are Skip to main content. In the previous article we discussed the specific heat capacity of substances.
Model 6200 is a microprocessor controlled isoperibol oxygen bomb calorimeter which is widely used for both routine and occasional calorific tests. Calorimeters have been designed in great variety. The calculated value does not match exactly but it is in the correct order of magnitude.
Dieter Haemmerich in Principles and Technologies for Electromagnetic Energy Based Therapies 2022. After the reaction the final temperature is 328C. Rise in temp ΔT 310250 K 60 K.
The entropy and the free energy of formation J. The temperature of the calorimeter rises from 250 to 310 K. A calorimeter is an object used for calorimetry or the process of measuring the heat of chemical reactions or physical changes as well as heat capacityDifferential scanning calorimeters isothermal micro calorimeters titration calorimeters and accelerated rate calorimeters are among the most common types.
Literature guides Concept explainers Writing guide Popular textbooks Popular high school textbooks Popular QA Business Accounting. DTA and DSC detect the temperature differences between the sample and the reference. Furthermore the chemical reaction such as thermal curing heat history specific heat capacity and purity analysis are also measurable.
Heat loss by the fuel is equal to the heat gained by the water. The only thing you need to remember is that you have to use consistent units for mass. The mass of the material m The temperature change that occurs DeltaT The specific heat capacity of the material c which you can look up.
The heat capacity of toluene from 14 deg K to 298 deg K. Mass of octane m 1750g. The definition of the calorie is based on the specific heat of water defined.
The water increases in temperature by 10 degrees C. And T f - T i is the change in temperature or the final temperature of the water minus the initial temperature of the water. 3234 Thermal capacity of calorimeter and water.
All data Tsonopoulos and Ambrose 1995. Such measurements can be made easily with this. 12744778 Latent heat capacity of steam.
Heat transferred to calorimeter Q mcΔT 000175 x 893 x 60 0938 kJ. First week only 499. Q water is the energy in the form of heat captured by the water expressed in calories cal.

50 Calorimetry Worksheet Answer Key Chessmuseum Template Library Worksheets Capacity Worksheets Letter Reversals

Constant Volume Calorimetry For More Precise Work Than The Coffee Cup Calorimeter The Heat Capacity Of The Entire C Coffee Cups Chemistry Education Chemistry

Pin By Redacted On Chemistry Education Chemistry Education What Is Science Ap Chem

Specific Heat Capacity Physics Lessons Science Teaching Resources Science Facts
No comments for "Heat Capacity of Calorimeter"
Post a Comment